Kinetic Molecular Theory
Temperature to Molecules
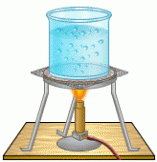
This part of the Kinetic Molecular Theory states that when heated, molecules get excited move more bouncing off of the walls of it containers, and eventually becomes a gas. For example, in the picture to the left, imagine the bubble as molecules, and the molecules are moving so much, they're coming out of the liquid state, and becoming a gas.
The Three States of Matter

-Solids (left) retain it's own shape, and the molecules stick firmly together. Since molecules are in constant motion, they are always vibrating, or bending.
-Liquids (middle) retains the shape of the container it's held in, but the molecules are moving randomly and gliding over each other.
-Gases (right) Does not retain a shape but it's molecules move in straight lines. The molecules bounce of each other, and the walls of its container, but no energy is lost.
-Liquids (middle) retains the shape of the container it's held in, but the molecules are moving randomly and gliding over each other.
-Gases (right) Does not retain a shape but it's molecules move in straight lines. The molecules bounce of each other, and the walls of its container, but no energy is lost.
