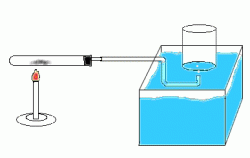
Dalton's Law of Partial Pressure
PressureTotal = Pressure1 + Pressure2 ... PressureN
Dalton's law of partial pressure describes total pressure on something, is the absolute pressure of everything combined. For example if you have 3.17kPa of water and 99.23kPa of hydrogen, you would get 102.4kPa of total pressure. Or in the picture below, you can see the pressure of the air, combined with the pressure of the substance in the testube pushing against the liquid.